Trifluoromethanesulfonic Anhydride
Product Details:
X
Product Description
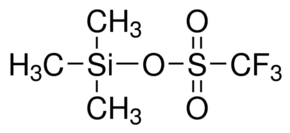
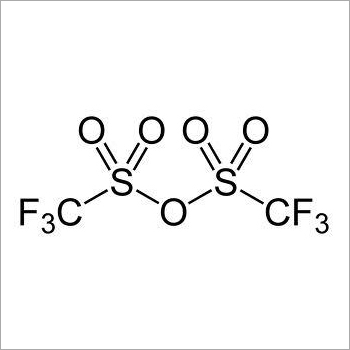
Trifluoromethanesulfonic anhydride, often abbreviated as Tf2O or Tf2O2S, is a chemical compound with the molecular formula CF3SO2O2S. It is also known by its IUPAC name, trifluoromethanesulfonyl trifluoromethanesulfonate. This compound is widely used as a strong and versatile electrophilic trifluoromethylating agent in organic synthesis.
Here are some key points about trifluoromethanesulfonic anhydride:
1. Structure: The compound has a structure consisting of two trifluoromethanesulfonyl (CF3SO2) groups linked by an oxygen atom.
2. Reactivity: Tf2O is a powerful electrophile due to the electron-withdrawing trifluoromethanesulfonyl groups. It is often used to introduce trifluoromethyl groups into organic molecules.
Safety Precautions:
Like many reactive chemicals, trifluoromethanesulfonic anhydride should be handled with care, and appropriate safety precautions should be taken. It is important to be aware of the potential hazards and to use proper protective equipment when working with this compound.
Trifluoromethanesulfonic Anhydride Properties:
1. Molecular Formula: The molecular formula of trifluoromethanesulfonic anhydride is CF3SO2O2S, indicating the presence of three fluorine atoms and two sulfonyl (SO2) groups.
2. Physical State: Tf2O is typically a colorless to pale yellow liquid at room temperature. Its physical state may be influenced by factors such as temperature and pressure.
3. Boiling Point: Trifluoromethanesulfonic anhydride has a relatively high boiling point, typically around 105-110 degree centigrade.
4. Reactivity: One of the notable properties of Tf2O is its high reactivity as a strong electrophile. It readily reacts with nucleophiles, making it a valuable reagent for introducing trifluoromethyl groups into organic molecules through triflation reactions.
5. Trifluoromethylating Agent: Tf2O is widely used as a trifluoromethylating agent in organic synthesis. The trifluoromethyl (CF3) group is often desirable in pharmaceutical and agrochemical compounds due to its unique electronic and steric properties.
6. Solubility: Trifluoromethanesulfonic anhydride is typically soluble in common organic solvents such as dichloromethane, chloroform, and ethyl acetate. Its solubility characteristics contribute to its use in various reaction conditions.
7. Electrophilic Nature: The compound's electrophilic nature arises from the presence of the electron-withdrawing trifluoromethanesulfonyl groups. This property makes it effective in activating carbon atoms for nucleophilic attack in chemical reactions.
8. Handling and Safety: Due to its reactivity, trifluoromethanesulfonic anhydride should be handled with care. Proper safety precautions, including the use of personal protective equipment and adherence to standard laboratory practices, are essential.
Trifluoromethanesulfonic Anhydride FAQ:
1. What is Trifluoromethanesulfonic Anhydride (Tf2O)?
Ans: Trifluoromethanesulfonic Anhydride, commonly abbreviated as Tf2O, is a chemical compound with the molecular formula CF3SO2O2S. It is a reagent used in organic synthesis, particularly as a strong electrophilic trifluoromethylating agent.
2. What is its role in organic synthesis?
Ans: Tf2O is used to introduce trifluoromethyl groups into organic molecules through a process called triflation. Trifluoromethyl groups can impart unique properties to organic compounds, making them valuable in pharmaceutical and agrochemical research.
3. How does Tf2O react in organic reactions?
Ans: Trifluoromethanesulfonic anhydride is highly reactive due to its electrophilic nature. It readily reacts with nucleophiles, facilitating the incorporation of trifluoromethyl groups into various organic substrates.
4. What are some applications of Tf2O in chemistry?
Ans: Tf2O finds applications in the synthesis of pharmaceuticals, agrochemicals, and other organic compounds where the introduction of trifluoromethyl groups is desired. It is a versatile reagent in the field of medicinal chemistry.
5. Is Tf2O hazardous to handle?
Ans: Yes, trifluoromethanesulfonic anhydride should be handled with care. It is important to follow proper safety precautions, including the use of personal protective equipment and adherence to standard laboratory practices.
6. What are the typical physical properties of Tf2O?
Ans: Trifluoromethanesulfonic anhydride is typically a colorless to pale yellow liquid with a relatively high boiling point, usually around 105-110 degree centigrade.
7. Can Tf2O be used in large-scale industrial processes?
Ans: While it is commonly used in laboratory-scale organic synthesis, the use of Tf2O in large-scale industrial processes may be limited due to safety considerations and the specialized nature of its applications.
8. Are there alternatives to Tf2O for trifluoromethylation reactions?
Ans: Yes, there are alternative reagents for trifluoromethylation reactions, but Tf2O is valued for its efficiency and versatility in introducing trifluoromethyl groups.
9. Where can I find more information about Tf2O and its use in organic synthesis?
Ans: Literature sources, scientific journals, and chemical databases are valuable resources for detailed information on the properties, reactions, and applications of trifluoromethanesulfonic anhydride.
10. Can Tf2O be stored for an extended period?
Ans: It is recommended to store Tf2O in a cool and dry place, away from moisture and incompatible materials. Storage conditions may vary, and it is crucial to follow guidelines provided by suppliers or regulatory agencies.
Enter Buying Requirement Details
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese