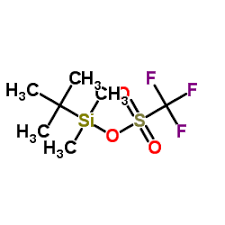
Triflic Anhydride
Product Details:
- CAS No 358-23-6
- Molecular Weight 282.14 Grams (g)
- Product Type Trifluoromethanesulfonic anhydride
- Physical Form Liquid
- Purity 98%
- Storage Room Temperature
- Application Technical
- Click to view more
X
Triflic Anhydride Price And Quantity
- 500 Kilograms
Triflic Anhydride Product Specifications
- Technical
- 1.677 Gram per millilitre (g/mL)
- 98%
- Room Temperature
- Liquid
- Trifluoromethanesulfonic anhydride
- 282.14 Grams (g)
- 358-23-6
Triflic Anhydride Trade Information
- 1000 Kilograms Per Week
- 1 Week
Product Description
Triflic anhydride is a very effective chemical molecule that has established itself as a crucial instrument in labs and research institutions all around the world. Due to its special qualities, it is an unavoidable option for a variety of applications, including organic synthesis and catalysis. Triflic anhydride is a preferred reagent for difficult conversions due to its unmatched reactivity. This adaptable substance is effective in a wide range of processes, including acylation, esterification, and olefination, to mention a few.
Triflic Anhydride Properties:
1. Chemical Formula: (CF3SO2)2O
2. Molecular Weight: Approximately 282.13 g/mol
3. Appearance: Triflic anhydride is a colorless to pale yellow liquid.
4. Odor: It has a strong, pungent odor.
5. Solubility: Triflic anhydride is highly soluble in common organic solvents such as dichloromethane, chloroform, and ether.
6. Reactivity: It is a powerful electrophile due to the trifluoromethylsulfonyl (triflyl) group. It reacts with nucleophiles, enabling various synthetic transformations, including trifluoromethylation reactions.
Triflic Anhydride Applications:
Trifluoromethylation Reactions: Triflic anhydride is widely employed for introducing trifluoromethyl groups (CF3) into organic molecules. The trifluoromethyl group can enhance the properties of compounds in medicinal chemistry, improving their bioavailability and metabolic stability.
Electrophilic Trifluoromethylation: It acts as a strong electrophile due to the trifluoromethylsulfonyl (triflyl) group, allowing it to react with various nucleophiles in synthetic transformations.
Catalyst Activation: Triflic anhydride is used as a catalyst activator, particularly in Lewis acid catalysis. It can activate Lewis acids such as metal triflates, facilitating reactions in organic synthesis.
Polymerization Reactions: It is employed in certain polymerization reactions, contributing to the development of specialty polymers with unique properties.
Dehydrating Agent: Triflic anhydride can serve as a dehydrating agent in the synthesis of various compounds, helping to remove water from reaction mixtures.
Catalyst for Esterifications: It can act as a catalyst in esterification reactions, promoting the formation of esters from carboxylic acids and alcohols.
Deoxyfluorination Reactions: Triflic anhydride is utilized in deoxyfluorination reactions, where it can replace oxygen atoms with fluorine atoms in organic molecules.
Amination Reactions: It has been employed in amination reactions, contributing to the synthesis of amines.
Olefin Sulfonation: Triflic anhydride is used in certain sulfonation reactions, where it can sulfonate olefins, leading to the formation of sulfonic acid derivatives.
Storage:
It is often stored in a cool, dry place, away from moisture and incompatible materials. Proper ventilation is important due to its strong odor.
Safety Precautions:
Triflic anhydride is a corrosive and highly reactive compound. Handling should be done with appropriate protective equipment, including gloves and eye protection. It is advisable to work in a well-ventilated area or under a fume hood.
Triflic Anhydride FAQ:
1. What is Triflic Anhydride?
Ans: Triflic anhydride, also known as trifluoromethanesulfonic anhydride (Tf2O), is a chemical reagent commonly used in organic synthesis. It is recognized for its role as a powerful trifluoromethylating agent in various chemical reactions.
2. What is its Chemical Formula?
Ans: The chemical formula for triflic anhydride is (CF3SO2)2O
3. What are its Properties?
Ans: Triflic anhydride is a colorless to pale yellow liquid with a strong, pungent odor. It is highly soluble in organic solvents, reactive as an electrophile, and requires careful handling due to its corrosive nature.
4. How is it Used in Organic Synthesis?
Ans: Triflic anhydride is employed for introducing trifluoromethyl groups into organic molecules, which can enhance their properties in medicinal and agrochemical applications. It serves as an electrophile in various synthetic transformations.
5. What Safety Precautions should be taken?
Ans: Triflic anhydride is corrosive and reactive. Proper protective equipment, including gloves and eye protection, should be worn. Work should be conducted in a well-ventilated area or under a fume hood. Safety data sheets (SDS) should be consulted for specific guidelines.
6. In what Reactions is it Commonly Used?
Ans: Triflic anhydride is commonly used in trifluoromethylation reactions, catalysis activation, polymerization, dehydrating reactions, esterifications, deoxyfluorination, amination, olefin sulfonation, and more.
7. Can it Replace Triflic Acid in Reactions?
Ans: Triflic anhydride can often replace triflic acid in reactions requiring the triflate (CF3SO3-) group. However, the choice between the two depends on the specific requirements of the reaction and the desired properties of the end product.
8. How is it Stored and Handled?
Ans: Triflic anhydride is typically stored in a cool, dry place away from moisture and incompatible materials. Proper ventilation is crucial. It should be handled with care, and safety guidelines for corrosive and reactive compounds should be followed.
9. What is its Role in Lewis Acid Catalysis?
Ans: Triflic anhydride is used as a catalyst activator in Lewis acid catalysis, helping to enhance the reactivity of certain catalysts in organic synthesis.
10. Is it Used in Pharmaceutical Research?
Ans: Yes, triflic anhydride is employed in pharmaceutical research for the synthesis of drug candidates and intermediates. The introduction of trifluoromethyl groups can influence the pharmacological properties of molecules.
FAQs of Triflic Anhydride:
Q: What is the physical form of Triflic Anhydride?
A: Triflic Anhydride is in liquid form.Q: What is the application of Triflic Anhydride?
A: Triflic Anhydride can be used for technical purposes and other applications.Q: What is the recommended storage condition for Triflic Anhydride?
A: Triflic Anhydride should be stored at room temperature.Q: What is the purity of Triflic Anhydride?
A: Triflic Anhydride has a purity of 98%.Q: What is the molecular weight of Triflic Anhydride?
A: The molecular weight of Triflic Anhydride is 282.14 grams.Q: What is the CAS number of Triflic Anhydride?
A: The CAS number of Triflic Anhydride is 358-23-6.Q: What is the density of Triflic Anhydride?
A: The density of Triflic Anhydride is 1.677 grams per millilitre.Enter Buying Requirement Details
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese